Robotic Research innovates to help fight COVID-19

D.C.-area engineering firm produces reusable face shields for region’s hospitals, offers sterile 3D printer patent for COVID applications globally
The team of engineers at Robotic Research LLC, based in Clarksburg, Maryland, is applying its innovative engineering expertise to create new solutions for healthcare workers to help fight the COVID-19 crisis in the Washington area and around the world.
Robotic Research is a provider of autonomy and robotic technologies for government and commercial customers.
The company has created a unique design for reusable face shields and is providing the shields to healthcare teams in hospitals around the Greater Washington Metro area. The company also just opened up its patented design of its sterile 3D printer by waiving licensing fees globally for any application of the printers related to fighting the current public health pandemic.
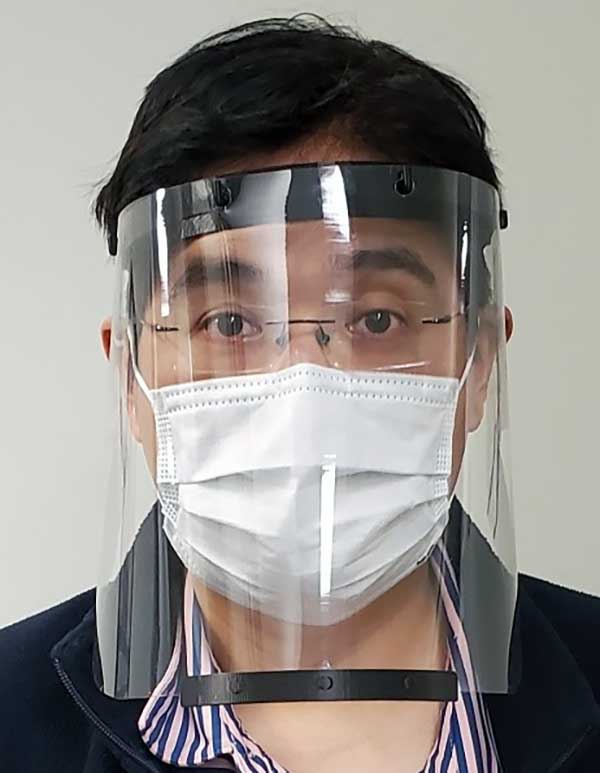
Face shields
The face shields, which Robotic Research is printing on standard industrial 3D printers at its robotics engineering facility in Clarksburg, are being delivered to support teams at hospitals in the Montgomery County area.
The face shield design provides comfort and protection, and features an improved disinfection process capability using materials compatible with hospital cleaning methods. The design has been approved for use in select departments, with broader uses under evaluation.
“Our team wanted to help out in any way we could to support the local frontline health-care providers who are working around the clock to assist those impacted by the COVID-19 public health crisis,” said Alberto Lacaze, president of Robotic Research. “We are producing a face shield that modifies a design based off the blueprint provided by the National Institutes of Health. The design includes some enhancements to further protect from aerosol in the temple area and allow the masks to be cleaned and therefore reusable, as well as comfortable to wear over prolonged use.”
Robotic Research has sourced materials from across the country to support the production of approximately 2,000 face shields. The company is seeking a grant through Montgomery County and the state of Maryland to boost production to approximately 500 shields a day. The company normally uses its standard 3D printers to support many of its robotic products and solutions.
Sterile 3D printer
Materials used for polymer 3D printing are often damaged by the high temperatures, chemicals or radiation used in standard sterilization processes. Robotic Research’s patented process overcomes this challenge by using a sterile chamber around a 3D printer where unsterilized plastic is brought into the chamber.
The possibly contaminated plastic is sterilized by the printing process and packaged maintaining the sterile field.
This system was initially designed for a U.S. government agency to allow sterile medical materials to be printed at locations where commonly used sterilization methods might not be possible.
“Because this printer can create sterile materials, which are sterile from the inside out once produced, we are eliminating the need for hospitals to re-sterilize equipment before use,” Lacaze said. “At a time when efficiency and supplies are critical, we hope this technology will be able to help alleviate some of the immense pressure hospitals and medical facilities are currently facing. It is our hope that providing the use of this process at no cost may help bring solutions to the health care teams working to save lives during this crisis.”
The unique printer process enables the manufacturing of sterile medical equipment and implantable device components, such as intubation tubes and other materials required during invasive procedures, in a manner that eliminates the need for additional sterilization.
Robotic Research’s patented sterile 3D printing process (USPTO 10,406,758) will be available royalty-free for a minimum of one year for any COVID-19 applications across the globe.
The company is committed to continuing to use its resources and facilities to develop solutions for the ongoing challenges of the COVID-19 pandemic.
















Follow Us